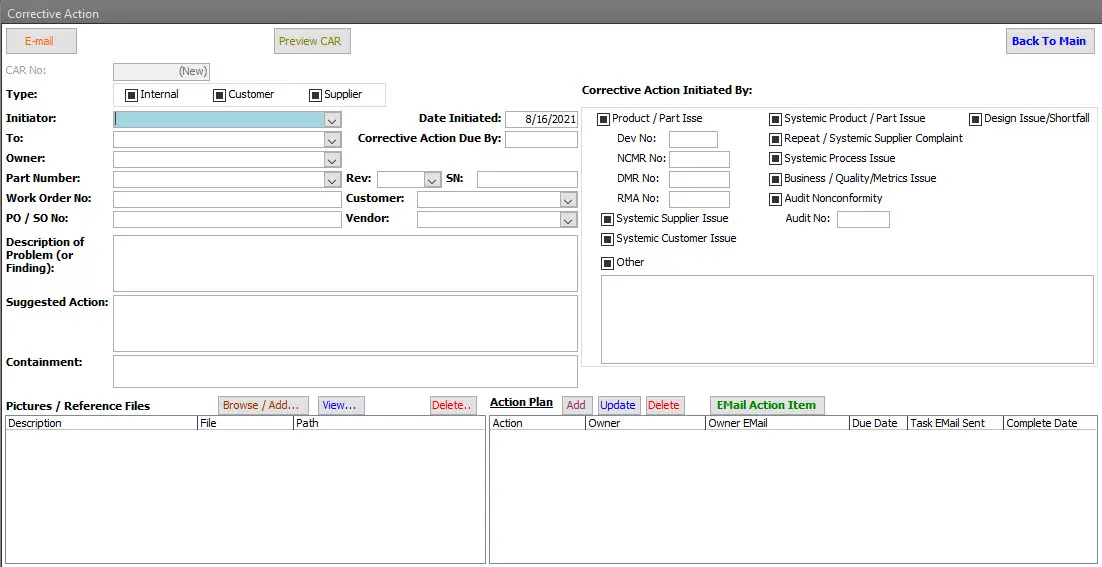
Corrective Action & Root Cause Analysis (RCA)
Corrective Action is a structured process that lets manufacturers identify, address, and eliminate issues like defects, inefficiencies, or safety hazards. With support for 5 Why root cause analysis, immediate actions, preventive strategies, and verification, it's built for robust ISO9001 & AS9100 compliance.

Core Features
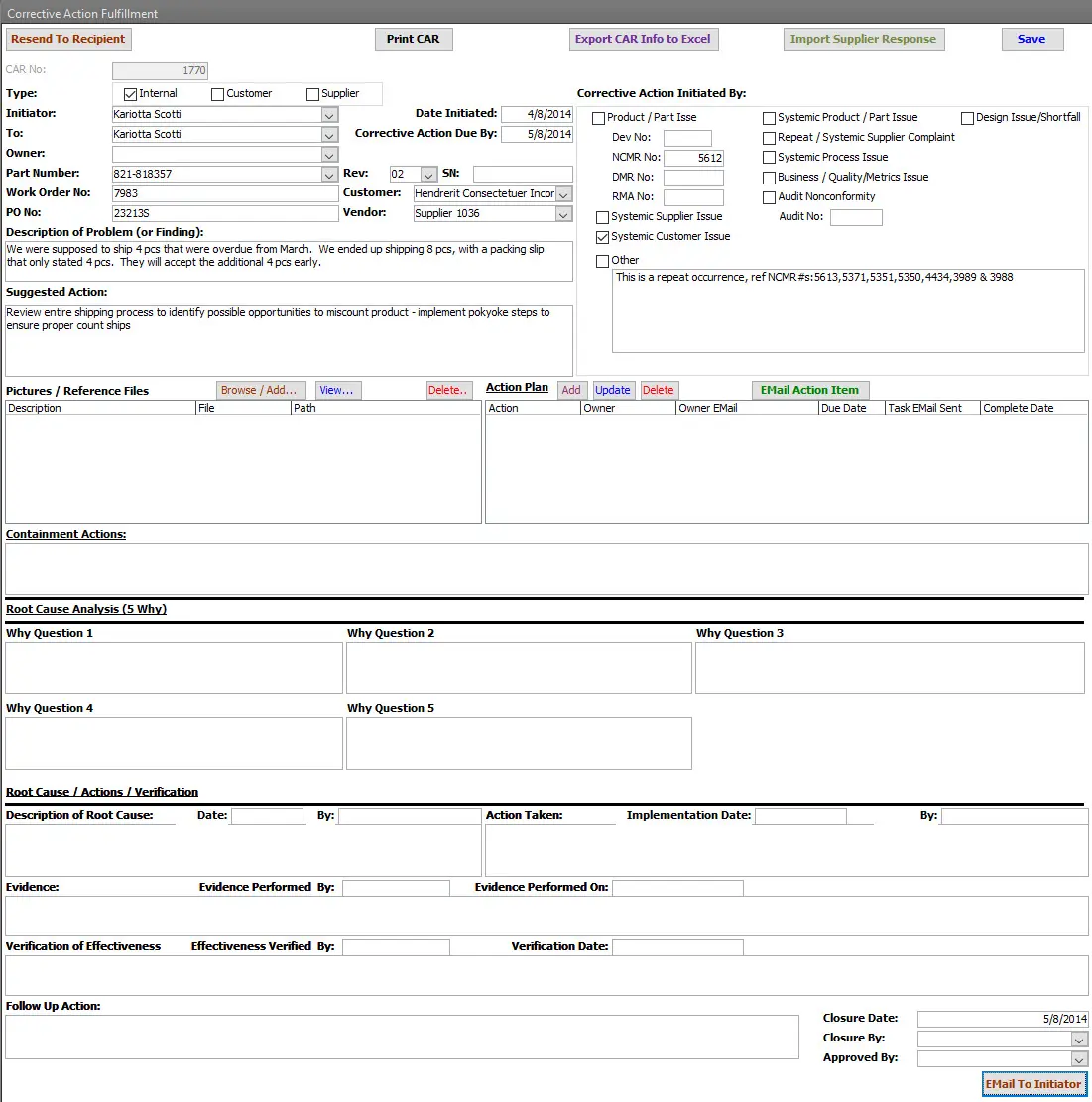
Root Cause Analysis (RCA) / 5 Why
Structured analysis to uncover underlying causes of problems and prevent recurrence.
- 5 Whys method built in
- Assign RCA owner and timeline
- Fishbone or other cause mapping optionally supported
Immediate Corrective Actions
Quick fixes to contain or control issues immediately while long-term corrective solutions are developed.
- Create temporary containment or mitigation
- Prevent immediate customer impact
- Track and escalate urgent issues
Preventive Actions & Process Improvement
Identify potential issues before they occur and continuously improve processes.
- Process reviews and audits to uncover risk
- Preventive workflows for trending issues
- Integration with lean / Six Sigma methods
Action Plan & Assignments
Document planned steps, responsibility, timelines, and actions taken across the corrective action process.
- Define who is responsible for each action
- Set due dates, track status
- Notify owners and stakeholders
Documentation & Communication
Keep a full record of issues, investigations, evidence, and outcomes, plus notify relevant parties.
- Link files: drawings, specs, nonconformances, emails
- Supplier response import for external CAPA
- Single-click emails for status, verification, closure
Verification & Effectiveness
Ensure corrective actions are implemented properly and actually resolve the root causes.
- Verify that actions were taken
- Measure effectiveness over time
- Record evidence and close out actions
Filtering & Context Linking
Provide context by linking corrective actions to orders, customers, suppliers, and related modules.
- Include part number, supplier, work order, sales order when applicable
- Use context to support trend analysis
Watch Our Corrective Action Processing Demo
Discover how SimpleManufacturing™ Corrective Action module empowers manufacturers to find and eliminate the root cause of problems—fast. This short demo walks you through every step:
- Launch a 5-Why Root Cause Analysis (RCA) with just a few clicks.
- Assign tasks and track progress for immediate and preventive actions.
- Maintain full documentation to stay ISO 9001 and AS9100 compliant.
- Verify effectiveness and prevent future nonconformances.
Whether you need to reduce defects, boost customer satisfaction, or streamline audits, this video shows how our intuitive software makes Corrective Action management simple, traceable, and audit-ready.
Call Now for a Free Demo: 858-335-6421
Request a Free DemoAdapted from SimpleManufacturing Corrective Action module. ([simplemanufacturing.com](https://www.simplemanufacturing.com/corrective-action/))

